Synergistic Effect
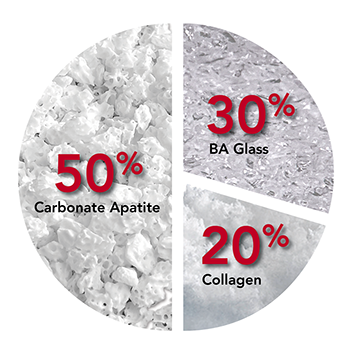
- An enhanced bone graft substitute composed of 45S5 bioactive glass, carbonate apatite anorganic bone mineral, and Type I collagen
- A combination that drives a synergistic effect to induce osteoblasts proliferation, matrix maturation, and extracellular matrix mineralization 1
Bioactive Glass
- 30% bioactive glass
- Promotes cell proliferation & differentiation 2
- 100-300pm particle Size 3,4,5
Carbonate Apatite
- Optimal resorption & remodeling-similar to human bone 6,7
- Pores provide pathways for cell migration and attachment to lay down new bone
- Higher osteoclastic & osteoblastic activity than BTCP & HA 8
Type 1 Collagen
- Highly absorbent, moldable, flexible, and resists migration upon irrigation
- Binds proteins and cells and retains biological factors 9
- 100% resorbable through normal metabolic pathways 10
- Intrinsic hemostatic properties control minor bleeding 10,11