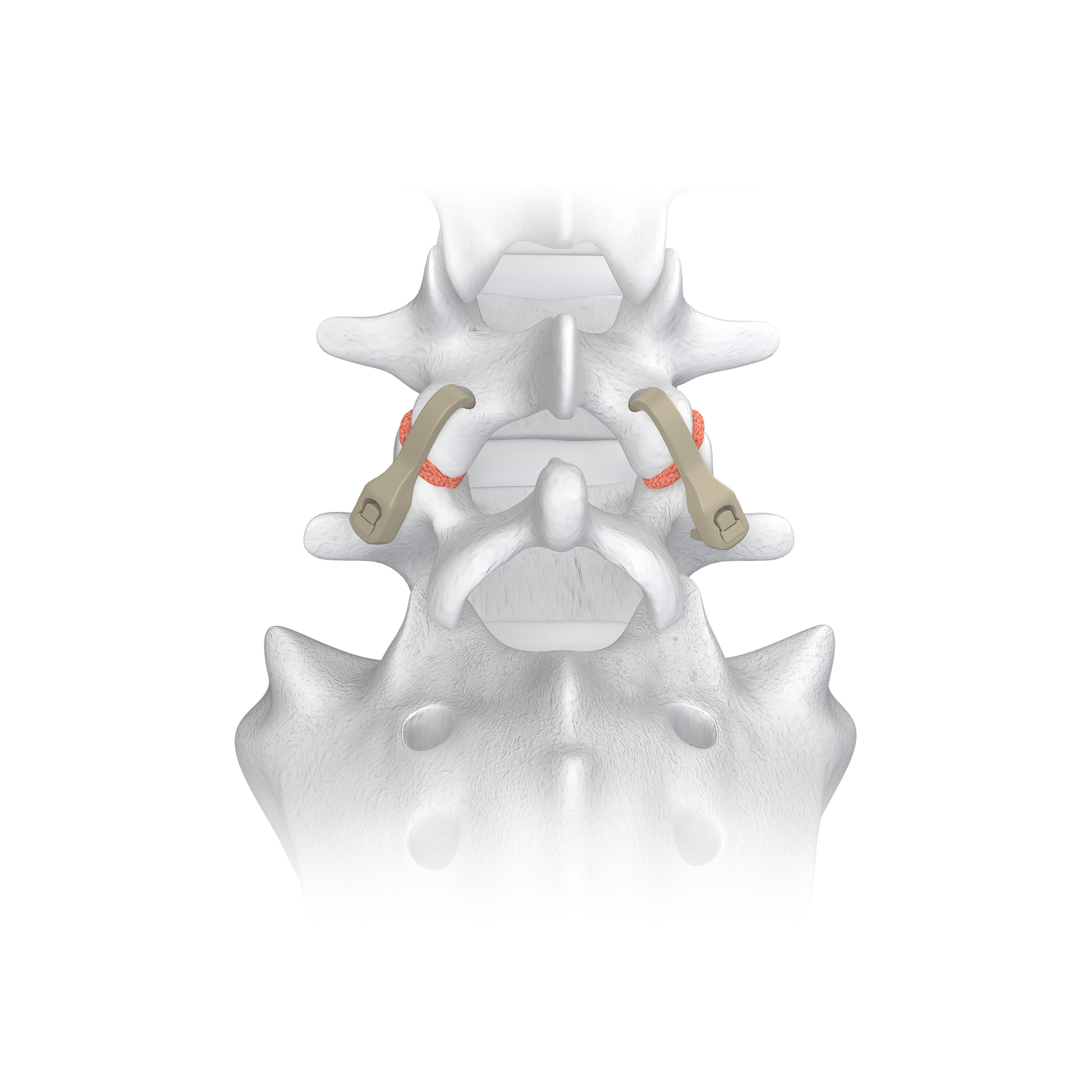
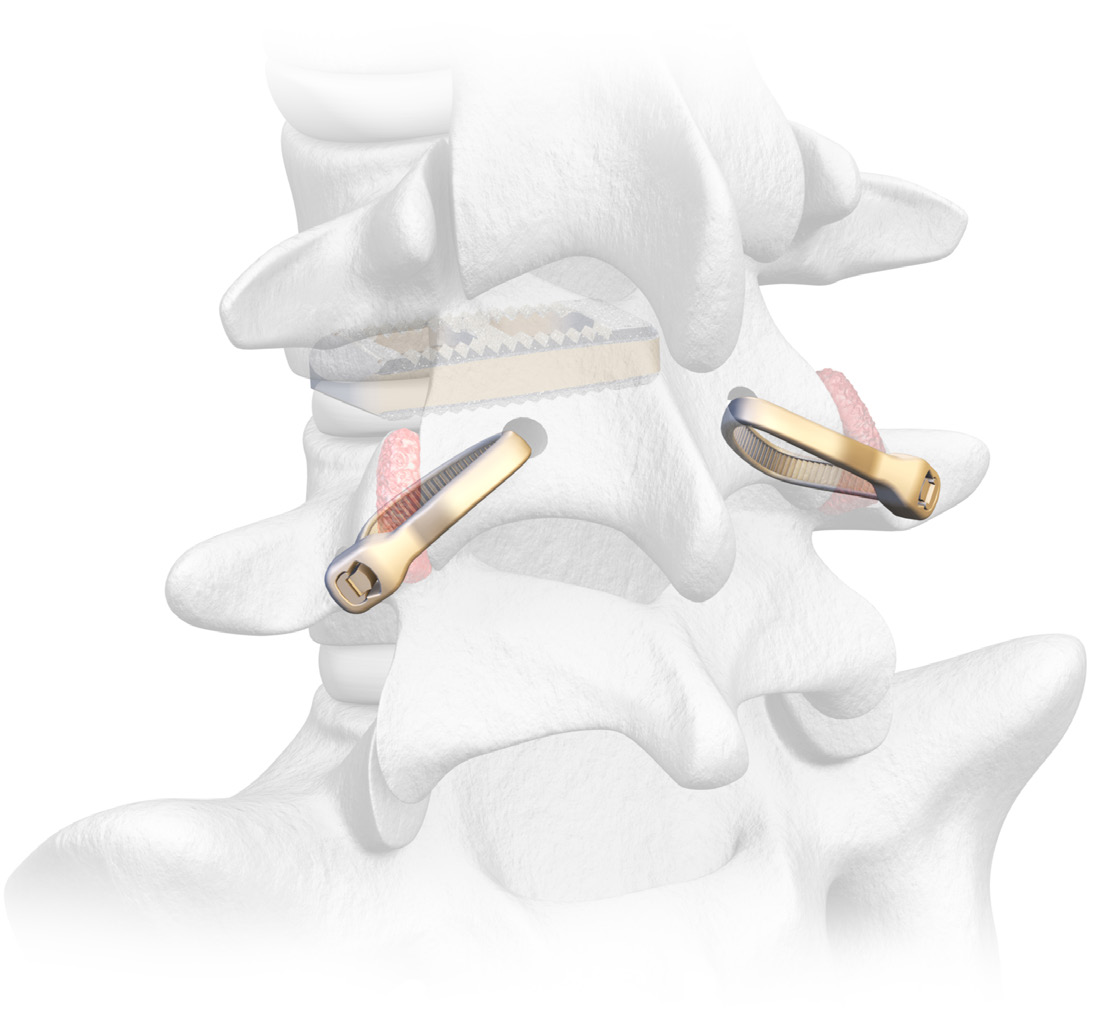
Spinal Elements® Announces First Publication on Karma® Metal-Free Cortico-Pedicular Posterior Fixation System
In this month’s World of Neurosurgery: Nunley, et al published “Metal-Free Cortico-Pedicular Device for Supplemental Fixation in Lumbar Interbody Fusion”
Carlsbad, CA, April 20, 2023 – Spinal Elements, a spine technology company, announced the first publication of the Karma Posterior Fixation system, describing preclinical and initial clinical results of metal-free cortico-pedicular fixation device used for posterior fixation and adjunct to fusion.
“On behalf of our co-authors*, I am very pleased to share this peer-reviewed publication on the safety, reproducibility, and biomechanics of the Karma MIS system,” states Pierce Nunley M.D., Orthopedic Surgeon, Director of the Spine Institute, Shreveport, LA. “Karma is a disruptive and patient-centric fixation alternative for degenerative disc disease. The FEA (Finite Element Analysis) data demonstrated similar stability while reducing stress shielding through the anterior column of the spine. My patients who have been treated using Karma have had very positive results.”
Additionally, the initial clinical experience presented in this paper has shown a 53% decrease in pain severity (P= 0.009), a 50% decrease in Oswestry Disability Index (P<0.001), and no device-related complications.
Karma is a metal-free cortico-pedicular posterior fixation system. Karma minimizes tissue trauma without the use of screws and rods while providing similar fixation to a pedicle screw system in degenerative spinal conditions. The Karma MIS system provides a simple reproducible procedure using a single set of instruments and a universal PEEK strap implant. Karma’s extremely low-profile implant compares favorably versus larger metal posterior fixation devices. Karma is part of Spinal Elements’ MIS Ultra® suite of products that are intended to address the unintended consequences of spine surgery.
Ron Lloyd, CEO of Spinal Elements, stated, “I am thrilled with the pre-clinical and the early clinical results of Karma. Even more inspiring though, has been the surgeon and patient experiences I have observed as we continue to expand the use of Karma to more and more healthcare institutions. We look forward to the broader release of the Karma MIS system in the coming weeks and months.”
About Spinal Elements
Spinal Elements is a Carlsbad, California-based medical device company focused on the design, development, and commercialization of a comprehensive portfolio of systems, products, and technologies for spine surgery procedures. A leading designer, developer, manufacturer, and marketer of innovative medical devices used in spinal surgical procedures, Spinal Elements combines leading medical device technologies, biologics, and instrumentation to create positive surgical outcomes that exceed surgeon and patient expectations. Spinal Elements has built a reputation delivering innovative and differentiated technologies that enable fundamental shifts in solutions for spine surgery. The company markets a complete portfolio of advanced spinal implant technologies.
For more information, please visit www.spinalelements.com.
*Authors: Pierce D. Nunley, Robert K. Eastlack, Larry E. Miller, Kornelis A. Poelstra, J Bridger Cox, Peter M. Shedden, Marcus Stone
For more information, contact:
Rick Simmons
Chief Marketing Officer
Spinal Elements
PRESS RELEASE MM-370-0022 Rev. 20230411